Abstract
Acute Myeloid Leukemia (AML) is a biologically diverse disease with subtypes that can be identified through integrated cytogenetic, mutational, and epigenetic characterization. Mutations in epigenetic regulators such as IDH1, IDH2, and DNMT3A are among the most common gene mutations found in AML (Cancer Genome Atlas Research et al. 2013; Papaemmanuil et al. 2016). Targeted inhibitors of IDH1 and IDH2 have recently been approved by the FDA for the treatment of AML (DiNardo et al. 2018; Stein et al. 2015). In addition, IDH/DNMT3A dual mutant AMLs exhibit a distinct epigenetic signature and are characterized by increased in vitro sensitivity to MEK inhibition (Glass et al. 2017).
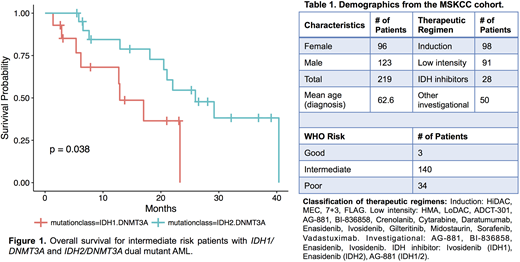
Here we explore the clinical course of AML patients with IDH1, IDH2, and DNMT3A mutations treated at Memorial Sloan Kettering Cancer Center (MSKCC) between 2012 and 2018. Treatment regimens included standard chemotherapy, as well as investigational therapies such as IDH1, IDH2, and pan-IDH inhibitors (Table 1). Using overall survival as our endpoint, we assessed contributions of WHO risk, age, treatment regimen, and gene mutations using multivariate Cox Proportional Hazards modeling. Among intermediate risk patients, we found that the presence of concurrent IDH1/DNMT3A mutations (n=16) conferred the highest mortality risk compared to DNMT3A mutation alone (HR=2.31; p=0.046). In contrast, IDH2/DNMT3A mutated AML conferred similar mortality risk compared to DNMT3A mutation alone (HR=1.07; p=0.85). When compared to intermediate risk IDH1/DNMT3A mutated AML, intermediate risk IDH2/DNMT3A mutated AML had a lower risk of mortality (HR=0.345; p=0.038), (Fig. 1). This adverse risk of IDH1/DNMT3A mutations persists after adjusting for IDH inhibitor therapy (HR=2.42; p=0.05). In multivariate analysis, IDH1 mutation and use of low intensity therapeutic regimens conferred an increased risk of mortality after adjusting for WHO risk (HR=2.25; p=0.016 and HR=1.71; p=0.036, respectively). Induction chemotherapy provided an overall survival benefit after adjusting for WHO Risk and the presence of IDH1, IDH2, DNMT3A, or dual IDH/DNMT3A mutations (HR=0.50; p<0.01). The increased risk associated with IDH1/DNMT3A dual mutations approached statistical significance after adjustment for WHO Risk and therapeutic modality (HR=2.00; p=0.08).
Overall, our findings suggest that AML with dual IDH1/DNMT3A mutations constitutes a higher risk disease entity. Further study of targeted treatment modalities such as dual IDH1/MEK inhibition or combined chemotherapy/IDH1 inhibition may be helpful in the treatment of these higher risk AML patients.
Levine:Isoplexis: Equity Ownership; Qiagen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Imago: Equity Ownership; Gilead: Honoraria; Janssen: Consultancy, Honoraria; C4 Therapeutics: Equity Ownership; Novartis: Consultancy; Epizyme: Patents & Royalties; Loxo: Consultancy, Equity Ownership; Prelude: Research Funding; Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Tallman:AbbVie: Research Funding; Cellerant: Research Funding; Orsenix: Other: Advisory board; BioSight: Other: Advisory board; AROG: Research Funding; Daiichi-Sankyo: Other: Advisory board; ADC Therapeutics: Research Funding. Goldberg:Abbvie: Research Funding; AROG: Research Funding; Pfizer: Research Funding; Celgene: Research Funding. Stein:Pfizer: Consultancy; Celgene: Consultancy; Bayer: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal